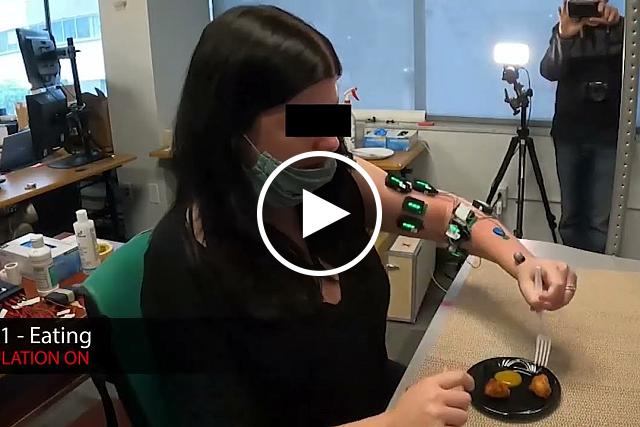You are here
July 9, 2024
Research in Context: Movement after paralysis
From electrical stimulation to robotics
NIH-funded researchers have been working to restore movement to people living with paralysis. This special Research in Context feature explores the different experimental approaches scientists are developing to allow people without control of their body’s movements to become more independent and improve their quality of life.

Most people may not pay much attention to the dizzying array of movements their bodies perform every day. Just in the morning, from sipping a cup of coffee to taking a shower and walking out the front door, the arms and legs execute a range of intricate motions in all directions.
But all that can change in the blink of an eye. Stroke, traumatic injuries, brain tumors, and infections can cause brain damage that affects movement and leads to paralysis.
Every year, around 800,000 people in the United States have a stroke. Stroke can damage any part of the brain, including areas that control movement. Many people never recover all, or even any, of their body’s capabilities afterwards.
Damage to the spinal cord can lead to paralysis in some or most of the body. More than a quarter-million people in the U.S. live with a spinal cord injury. And many rare diseases and disorders such as amyotrophic lateral sclerosis (ALS) also cause different degrees of paralysis.
NIH-funded researchers have been working to restore movement to people living with paralysis. Many concepts that once sounded futuristic—such as using electrodes to amplify brain signals and harnessing the power of robotics—are now being tested in people.
"There are several very promising therapeutic technologies that are making their way through the early stages of clinical research," says Dr. Brooks Gross, a neurotechnology expert at NIH. While each of these technologies is unlikely to make someone regain all the functions lost to illness or injury, they may begin to allow people to be more independent and improve their quality of life.
Amplifying brain signals
Stroke can be caused by a blood clot or bleed in the brain. Either way, the sudden lack of life-giving oxygen to an area of the brain leads to rapid death of brain cells called neurons. In many people, this cell death occurs in the part of the brain that controls movement, called the motor cortex, explains Dr. Marco Capogrosso, a neuroscientist at the University of Pittsburgh.
Spinal cord stimulation allowed stroke patients to perform daily activities like eating with a fork. University of Pittsburgh
After a stroke, people may have muscle weakness or paralysis, especially in the arms and hands. This can interfere with their ability to perform even basic activities of daily living, like eating or grooming.
But after a stroke, Capogrosso explains, the neural circuits in the spine remain intact. This means those spinal circuits can be tapped into.
Capogrosso's team is one of many that has been working on a technique called spinal cord stimulation. They’ve implanted electrodes into areas of peoples’ spinal cords that are normally controlled by the motor cortex. When activated, these electrodes act like an amplifier for the weakened signals coming from the brain.
“After stroke, when the motor cortex is speaking, the sound is very feeble,” Capogrosso says. “What we’re doing is amplifying the capacity of the circuits below the damage to hear those signals, even if the sound is really faint.”
In a recent study, his team recruited two volunteers who had partial paralysis of their upper limbs after a stroke. Each received implants of two electrode arrays in the neck region of the spinal cord, called the cervical spine, for about a month.
The researchers found that continuous electrode stimulation, tailored for each participant, immediately improved the strength, function, and range of movement in the participants’ arms and hands.
Stimulation also enabled the return of some fine motor skills. With electrodes activated, participants could perform tasks that they hadn’t been able to do for years, such as opening a lock, eating with a fork, or grasping and lifting a can of soup.
Importantly, when the electrodes were removed, the researchers saw that some improvements remained for weeks afterward.
This lasting improvement points to what could turn out to be one of the main benefits of spinal cord stimulation, “which is to essentially amplify motor rehabilitation,” says Capogrosso. “If you receive stimulation and can do a lot of repetitions of an exercise, then when you go home, you’re hopefully a little better,” even with the stimulation off, he explains.
Boosting movement noninvasively
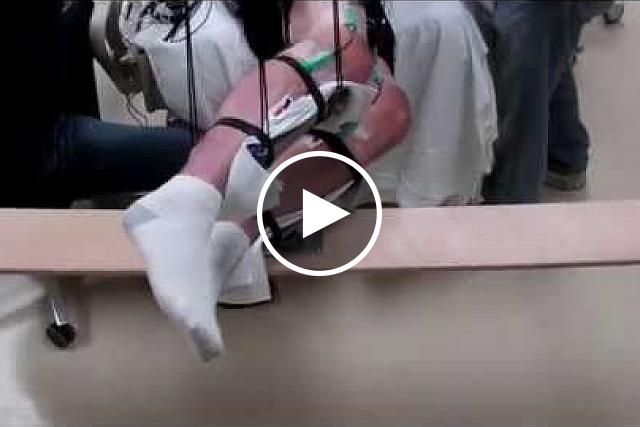
Other NIH-funded teams have tested spinal-cord stimulation without implanting electrodes. In this concept, called transcutaneous electrical nerve stimulation or TENS, electrical currents are delivered to the spinal cord using electrodes placed on the skin.
Video shows range of voluntary movement before and after transcutaneous stimulation. The subject's legs are supported so that they can move without resistance from gravity. The electrodes are used to record muscle activity. Edgerton laboratory / UCLA
When combined with intensive physical rehabilitation, such temporary stimulation to the part of the spinal cord controlling the upper limbs has resulted in long-term improvements in grip strength and the ability to perform daily activities like personal grooming. In the legs, the combination restored some participants’ ability to move their legs in a step-like pattern.
Neither of these two approaches—invasive or noninvasive stimulation—are commercially available yet. But together, they could have a wide range of applications.
While invasive stimulation requires surgery, it is typically more effective, bringing an immediate improvement in control when turned on. However, noninvasive stimulation doesn't require surgery, making it potentially more affordable and safer for some people.
Researchers are now also working on systems to help control functions inside the body. These include helping with bowel, bladder, and blood pressure control in people with paralysis.
For example, one NIH-funded team has been testing a spinal cord stimulation system to improve bladder control after a spinal cord injury. Another has developed a wireless implantable device that can monitor bladder filling in real time and send data to a smartphone.
“These can be big issues for people with spinal cord injury, and restoring them can help people be less dependent on others,” Gross says. Such systems are likely to be some of the first to become available to patients with paralysis, he adds.
Regrowing lost connections
For spinal cord stimulation to work, the spinal cord must still be able to relay signals from the motor cortex. In a spinal cord injury, the damage is to these receiver cells in the spine itself. Without such connections, messages from the brain that control movement are essentially disconnected.
“If a patient has lost function below the level of a spinal cord injury, they’ve lost more than 90% of the functional nerve connections passing across the injury site,” explains Dr. Mark Tuszynski, a regenerative medicine researcher at the University of California, San Diego.
But what if these connections could be regrown? “Healing does happen after a spinal cord injury,” Tuszynski explains. “But regeneration doesn’t. The immune response tends to wall off the damage to prevent it from spreading. But that often ends up being part of the problem preventing regeneration.”
His lab and others have been testing the strategy of flooding the injured spine with a type of stem cell that, under the right conditions, can grow into new neurons. Previous clinical trials of therapies to regrow nerve cells after spinal cord injury have largely shown disappointing results, Tuszynski says. But in those trials, people were trying to tap into the remaining 10% of healthy nerve cells, he explains. “They weren’t trying to promote regeneration of the cut nerve cells.”
By providing those cut nerve cells with cells to connect with, his team hopes to increase the number of cells that can route movement signals from the brain. To ensure the survival of these fragile new cells in the spine, the researchers encase them in a protective gel.
They’ve recently seen promising results of this approach in monkeys with a spinal cord injury. “Hopefully, in about two years, we’ll have enough data to begin human clinical trials,” he says.
His team has also tested a 3D-printed scaffold to support the cells and guide them to grow in specific patterns in the spine. Rats with spinal cord injury that received the scaffold plus neural stem cells regained some movement in their back leg joints by six months after injury. “We’re now testing this strategy in monkeys, to see whether it could represent the next generation of therapy,” he says.
In addition, his and many other labs have been experimenting with adding molecules that can potentially boost and guide the growth of new neurons from stem cells. “Maybe this will get us even more connections with neurons around the injury,” Tuszynski says.
Robotic help
While some researchers are focusing on restoring the connections between brain and body after paralysis, others are taking an entirely different approach to get people moving again: robotics.
One example of wearable robotic devices are exoskeletons, which can be worn to assist with walking or lifting. But due to high costs and large energy and computing requirements, exoskeletons have largely been limited to the laboratory environment.

Researchers created a robotic leg exoskeleton that provided personalized walking assistance under real-world conditions. Robotic exoskeletons could assist people with mobility impairments or with physically demanding jobs. Kurt Hickman
Recently, the world has seen a revolution in cheap, wearable sensors. Everything needed to make an exoskeleton work has become smaller and more powerful over the last decade, explains Dr. Scott Delp, a bioengineering researcher at Stanford University. “We can now outfit the body with sensors, with motors, with computers, and everything is self-contained.”
In a recent study, his team helped test a portable exoskeleton around the ankle that could rapidly adapt to the user during normal walking. The exoskeleton was powered by a battery pack worn at the waist. With each step, a motor provided torque at the ankle, helping the user to push off.
Using a tiny integrated computer, the exoskeleton was able to adapt to the individual’s gait in about an hour.
“We’ve now set out to collect the world’s largest database describing human movement that we can use to train an AI model to control our exoskeleton,” Delp says. This project is being led by one of Delp’s researchers, who himself lives with a progressive muscle disease. The goal of the AI model is to allow the exoskeleton to sense the intentions of muscles that can’t complete their movement because of paralysis, and use those intentions to guide it.
“In the case of a stroke or neuromuscular disease, people still have intentions that can be sensed and used to control the exoskeleton. That way the user is the pilot, instead of the other way around,” he says.
Combining approaches
While neural stimulation, regenerative medicine, and robotics may seem like wildly different fields, they’re eventually likely to be combined to provide the greatest possible rehabilitation after paralysis. “I think that being able to integrate all of these technologies is what’s going to have to happen for them to provide the greatest therapeutic benefit to patients,” Gross says.
Capogrosso and his team are already collaborating with regenerative medicine researchers. “You have to have some spare neural connections for spinal stimulation to work. If you don’t have them, what if we could boost them with regeneration techniques?” he asks. “Even just a little bit could get you to the threshold where, suddenly, we can use our technology.”
“We’re also looking at combining implanting stem cells with noninvasive stimulation,” Tuszynski says. “Ultimately, the best solution is likely to be a combination approach.”
Combining neural stimulation with an exoskeleton may also have potential to speed rehabilitation, Delp adds. “To get the most benefit from spinal cord stimulation, you have to have stimulation and be moving,” he explains. This is now done with intensive physical therapy but, he adds, “using an exoskeleton could potentially enable you to get many more repetitions, to better train those motor patterns during stimulation.”
With many of these technologies already being tested in clinical trials or growing closer to human studies, there also needs to be research on how they interact with each other and with other common treatments like pain medication, Gross says. “It’s important to look at the whole body, to increase the likelihood that the therapies we eventually provide are effective in as many patients as possible.”
—by Sharon Reynolds
Related Links
- Sensor Monitors Bladder Fullness
- Spinal Cord Stimulation Improves Arm, Hand Movements After Stroke
- Robotic Exoskeleton Helps People Walk
- Device Allows Paralyzed Man to Communicate with Words
- System Turns Imagined Handwriting into Text
- Printed Scaffolds Promote Spinal Cord Repair in Rats
- Regrowing Neurons Across Scarred Spinal Tissue
- Spinal Cord Stimulation Improves Hand Grip After Cervical Spinal Injury
- Spinal Cord Stimulation Helps Paralyzed People Move Hands
- Paralyzed Men Gain Movement Without Surgery
- Paralyzed Men Regain Movement with Spinal Stimulation
- Bionic Movements: Connecting Mind and Machine
- Stroke
- Spinal Cord Injury
- Tissue Engineering and Regenerative Medicine
- Rehabilitation Engineering
- The Brain Research Through Advancing Innovative Neurotechnologies® (BRAIN) Initiative
References:
Epidural stimulation of the cervical spinal cord for post-stroke upper-limb paresis. Powell MP, Verma N, Sorensen E, Carranza E, Boos A, Fields DP, Roy S, Ensel S, Barra B, Balzer J, Goldsmith J, Friedlander RM, Wittenberg GF, Fisher LE, Krakauer JW, Gerszten PC, Pirondini E, Weber DJ, Capogrosso M. Nat Med. 2023 Mar;29(3):689-699. doi: 10.1038/s41591-022-02202-6. Epub 2023 Feb 20. PMID: 36807682.
Noninvasive activation of cervical spinal networks after severe paralysis. Gad P, Lee S, Terrafranca N, Zhong H, Turner A, Gerasimenko Y, Edgerton VR. J Neurotrauma. 2018 Sep 15;35(18):2145-2158. doi: 10.1089/neu.2017.5461. PMID: 29649928.
Engaging cervical spinal cord networks to reenable volitional control of hand function in tetraplegic patients. Lu DC, Edgerton VR, Modaber M, AuYong N, Morikawa E, Zdunowski S, Sarino ME, Sarrafzadeh M, Nuwer MR, Roy RR, Gerasimenko Y. Neurorehabil Neural Repair. 2016 Nov;30(10):951-962. doi: 10.1177/1545968316644344. Epub 2016 May 18. PMID: 27198185.
Noninvasive reactivation of motor descending control after paralysis. Gerasimenko Y, Lu D, Modaber M, Zdunowski S, Gad P, Sayenko D, Morikawa E, Haakana P, Ferguson AR, Roy RR, Edgerton VR. J Neurotrauma.2015 Dec 15;32(24):1968-80. doi: 10.1089/neu.2015.4008. Epub 2015 Aug 20. PMID: 26077679.
Biomimetic 3D-printed scaffolds for spinal cord injury repair. Koffler J, Zhu W, Qu X, Platoshyn O, Dulin JN, Brock J, Graham L, Lu P, Sakamoto J, Marsala M, Chen S, Tuszynski MH. Nat Med.2019 Feb;25(2):263-269. doi: 10.1038/s41591-018-0296-z. Epub 2019 Jan 14. PMID: 30643285.
Required growth facilitators propel axon regeneration across complete spinal cord injury. Anderson MA, O'Shea TM, Burda JE, Ao Y, Barlatey SL, Bernstein AM, Kim JH, James ND, Rogers A, Kato B, Wollenberg AL, Kawaguchi R, Coppola G, Wang C, Deming TJ, He Z, Courtine G, Sofroniew MV. Nature. 2018 Sep;561(7723):396-400. doi: 10.1038/s41586-018-0467-6. Epub 2018 Aug 29. PMID: 30158698.
Personalizing exoskeleton assistance while walking in the real world. Slade P, Kochenderfer MJ, Delp SL, Collins SH. Nature. 2022 Oct;610(7931):277-282. doi: 10.1038/s41586-022-05191-1. Epub 2022 Oct 12. PMID: 36224415.
A wireless, implantable bioelectronic system for monitoring urinary bladder function following surgical recovery. Kim J, Bury MI, Kwon K, Yoo JY, Halstead NV, Shin HS, Li S, Won SM, Seo MH, Wu Y, Park DY, Kini M, Kwak JW, Madhvapathy SR, Ciatti JL, Lee JH, Kim S, Ryu H, Yamagishi K, Yoon HJ, Kwak SS, Kim B, Huang Y, Halliday LC, Cheng EY, Ameer GA, Sharma AK, Rogers JA. Proc Natl Acad Sci U S A. 2024 Apr 2;121(14):e2400868121. doi: 10.1073/pnas.2400868121. Epub 2024 Mar 28. PMID: 38547066.
First in human subjects testing of the UroMonitor: a catheter-free wireless ambulatory bladder pressure monitor. Frainey BT, Majerus SJA, Derisavifard S, Lewis KC, Williams AR, Balog BM, Butler RS, Goldman HB, Damaser MS. J Urol. 2023 Jul;210(1):186-195. Doi: 10.1097/JU.0000000000003451. Epub 2023 Jun 9. PMID: 37293725.